CARRE aims at researching and innovating towards a service environment for providing personalized empowerment and shared decision support services for cardiorenal disease comorbidities. CARRE evaluation during the final months of the project aims to assess the CARRE service along four different axes:
- the efficacy of CARRE service in increasing health literacy;
- the ability of the CARRE service to empower patients;
- the impact of the service on quality of life; and
- improvement of the medical condition of the patient.
The investigational protocol is designed as a randomized, single-blind, controlled pilot study. Primary objectives of the study are the following:
- to increase health literacy;
- to increase level of patient empowerment;
- to improve patient’s quality of life;
- to reduce the personal risk of cardiorenal disease related morbidities (as these are described in the CARRE risk factor database).
Secondary objectives of the study are the following:
- to ameliorate or prevent the progression of clinical and laboratory parameters related to cardiorenal disease and comorbidities;
- to improve lifestyle habits (smoking, physical activity, adherence to self-monitoring and therapy);
- to limit the number or dose of essential drugs;
- to test for intervention acceptability and/or user satisfaction.
Accurate measurement of health literacy is a critical component to identify topics and populations most in need of support [1]. Haun et al. [2] summarize and compare 51 instruments for Health literacy measurement. They identified 26 questionnaires which measure general health literacy, 15 which are disease specific and 10 which are related with specific population. Take to account the strengths and the limitations of the questionnaires, we concluded that a questionnaire which will be consisted of general questions about health literacy, using the European Health Literacy Questionnaire [3] enriched with questions from Lipkus Expanded Health Numeracy Scale [4] which is a questionnaire which indicate if patient perceive his/her health risk, is the most appropriate questionnaires combination for our study.
The assessment of patient empowerment in CARRE will be based on the instrument developed in the EU funded SUSTAINS project questionnaire [5]. The background to the SUSTAINS project has three drivers that SUSTAINS contributes to: a) enabling and strengthening empowerment of patients; b) enabling better medical results; c) enabling a more efficient use of healthcare resources and containing costs. The instrument developed in the project is available in many European languages, amongst them Greek and English.
In the field of healthcare, quality of life is often regarded in terms of how a certain ailment affects a patient on an individual level. This may be a debilitating weakness that is not life-threatening; life-threatening illness that is not terminal; terminal illness; the predictable, natural decline in the health of an elder; an unforeseen mental/physical decline of a loved one; or chronic, end-stage disease processes. The consortium decided to use SF-36, as it appears to be the most comprehensive and the most commonly used, while it is validated for both pilot languages (Greek [6] and Lithuanian [7]).
The usability of a system, as defined by the ISO standard ISO 9241 Part 11, can be measured only by taking into account the context of use of the system — i.e., who is using the system, what they are using it for, and the environment in which they are using it. Furthermore, measurements of usability have several different aspects: effectiveness (can users successfully achieve their objectives); efficiency (how much effort and resource is expended in achieving those objectives); and satisfaction (was the experience satisfactory)
There are many survey instruments available for the usability assessment of a product or service. System Usability Score (or SUS) is an easy and effective tool for assessing the usability of diverse products including hardware, software, mobile devices, websites and applications. SUS, initially developed by Brooke [8] has become an industry standard, with references in numerous publications. SUS is a reliable, low-cost usability scale that can be used for global assessments of systems usability [9,10].
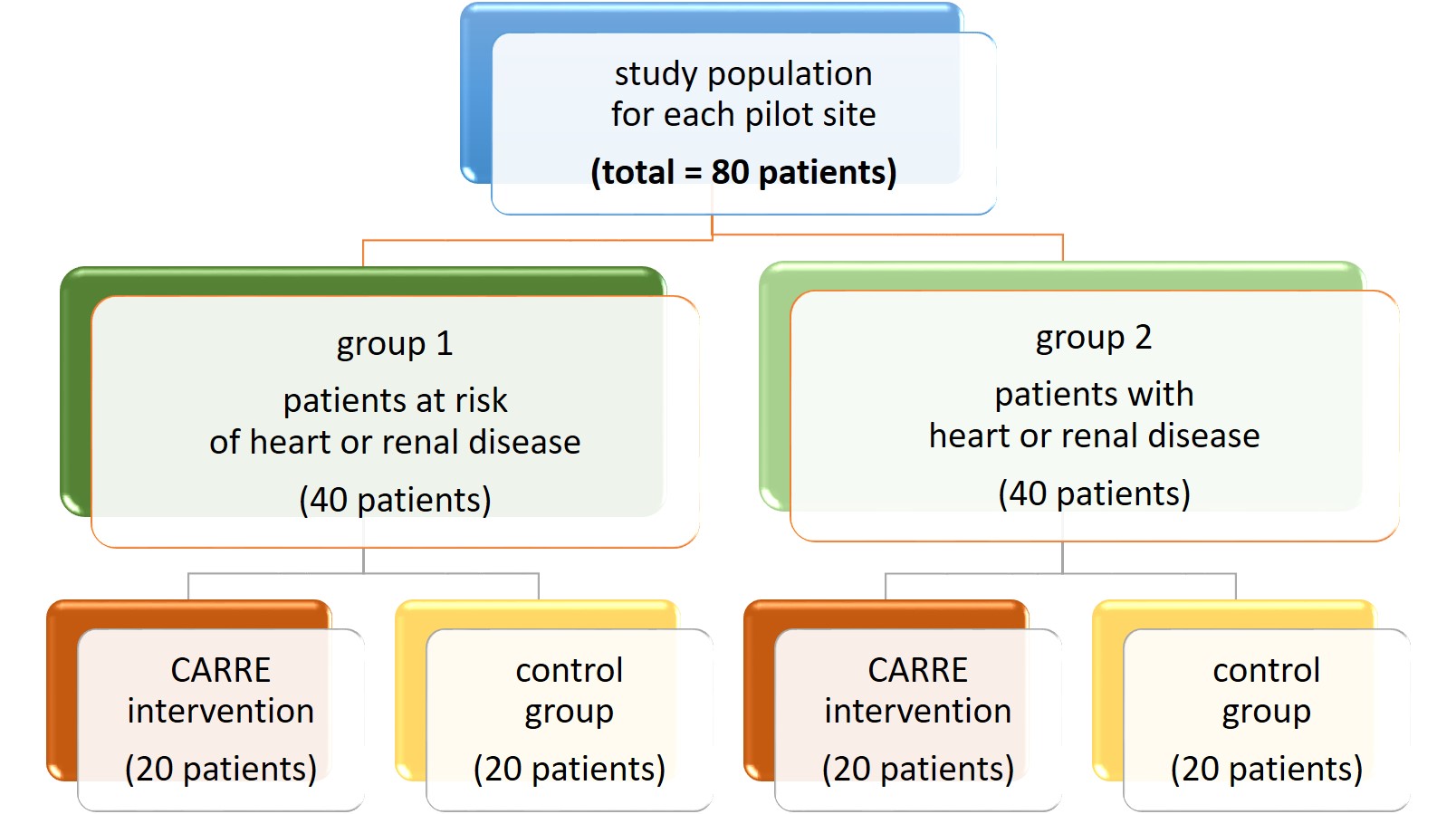
CARRE evaluation group (CARRE group) and Control group will be balanced by age, gender and number of patients with heart failure or chronic kidney disease (in Group 2). The same protocol will be used in 2 pilot sites. Overall the study expects to include 160 subjects: 80 subjects per pilot, for 2 pilot sites. For each pilot, two population groups will be assembled. Group 1: subjects with a diagnosis of metabolic syndrome and Group 2: Subjects with a diagnosis of either renal or heart disease, causing already diagnosed chronic kidney disease or chronic heart failure.
References:
- McCormack L, Haun J, Sørensen K, Valerio M (2013). Recommendations for advancing health literacy measurement. J Health Commun. 2013;18 Suppl 1:9-14. doi: 10.1080/10810730.2013.829892.
- Haunab, MA. Valerioc, LA. McCormackd, KSørensene & MK. Paasche-Orlowf, Health Literacy Measurement: An Inventory and Descriptive Summary of 51 Instruments, Journal of Health Communication: International Perspectives, Volume 19, Supplement 2, 2014
- Sorensen, S. V. den Broucke, J. M. Pelikan, J. Fullam, G. Doyle, Z. Slonska, B. Kondilis, V. Stoffels, RH Osborne, H. Brand. Measuring health literacy in populations: Illuminating the design and development process of the European Health Literacy Survey Questionnaire (HLS-EU-Q). BMC Public Health, 13, 948, 2013
- M. Lipkus, G. Samsa, & B. K. Rimer. General performance on a numeracy scale among highly educated samples. Medical Decision Making: An International Journal of the Society for Medical Decision Making, 21, 37–44, 2001
- Unver, W. Atzori, Document D3.2 – Questionnaire for Patient Empowerment Measurement Version 1.0, SUSTAINS: Support USers To Access INformation and Services, January 2013, EU CT PSP Grant Agreement No 29720
- Pappa, N. Kontodimopoulos, D. Niakas Validating and norming of the Greek SF-36 Health Survey. Qual Life Res. 2005 Jun;14(5):1433-8.
- Rugiene, J. Dadoniene, A. Venalis Adaptation of health-related quality of life (“SF-36”) questionnaire, its validation and assessment of performance for control group and patients with rheumatoid arthritis]. Medicina (Kaunas). 2005;41(3):232-9. Lithuanian.
- Brooke, (1996). SUS: a „quick and dirty‟ usability scale. In P.W.Jordan, B. Thomas, B.A. Weerdmeester, and I.L. McClelland (Eds.) Usability Evaluation in Industry (189-194). London: Taylor and Francis.
- Bevan, J. Kirakowski, and J. Maissel, What is Usability?, in H.-J. Bullinger, (Ed.). Human Aspects in Computing: Design and use of interactive systems and work with terminals, Amsterdam: Elsevier. 1991
- Kirakowski, and M. Corbett, Measuring User Satisfaction, in D M Jones and R Winder (Eds.) People and Computers IV. Cambridge: Cambridge University Press. 1988