Cardiorenal disease and comorbidities is a complex domain. Related conditions do not have a single cause, but evidence suggests that there are multiple causal chains. In order to capture this in CARRE, current evidence will be presented as a complex network of risk factors, that is, pairs of conditions one related to another via a causal relationship.
In medicine risk is the probability of a negative outcome on the health of a population of subjects. The agents responsible for that risk are called risk factors when they aggravate a situation and are being used to predict up to a degree the occurrence of a condition or deterioration of a patient’s health dividing the population into high and low risk groups. In general, risk factors can be:
- Environmental . It includes chemical, physical, mechanical, biological and psychosocial elements that constitute risk factors to public health.
- Demographic. Empirical findings have pointed out that age, sex, race, location, and religion all affect public health.
- Genetic. Any predisposition to conditions and habits hardcoded in the human gene.
- Behavioral – Lifestyle related. Human behaviors that are marked as “risky” and have proven to cause deterioration or provide added risk like smoking, overeating, unprotected sexual life, excessive alcohol drinking, drug abuse and sedentary lifestyle.
- Biomedical. These include conditions present in a patient that can influence his/her health by creating or affecting other conditions.
The relation between the two conditions, initial and resulting may not always be proven causation. Following UMLS Semantic Network, associations between a risk factor and the associated condition include:
- issue_in: the risk factor is a point of discussion for a condition
- affects: the risk factor produces a direct effect on the condition
- causes: the risk factor brings about the conditioncomplicates: the risk factor causes another (risk) factor to become more complex (recursive).
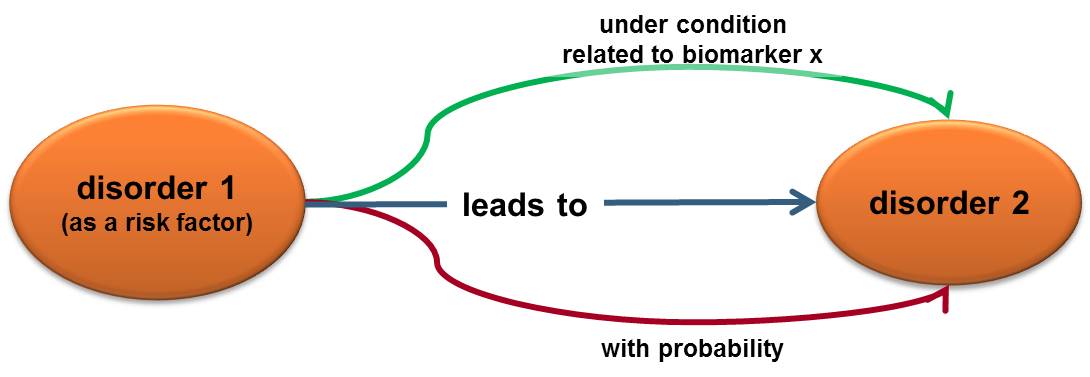
The existence of a risk factor is not a determinant of consequence but the degree of its influence can be statistically calculated. Thus, we can present a risk factor as a triplet:
1) what can happen (what is the event, factor/condition/disorder 1)
2) what are the consequences (what is the resulting condition/disorder 2)
3) what is the likelihood of having these consequences when the event is present.
The way to measure the likelihood requires a certain quantitative biomarker and observational studies that statistically calculate a probability. This probability is expressed as a risk ratio.
Relative risk or Risk ratio (RR) is the ratio of the probability of an event occurring (for example, developing a disease) in an exposed group to the probability of the event occurring in a non-exposed group.
Another metric of relative risk is the Hazard Ratio (HR) which is most often used in clinical studies to assess the instantaneous risk at any time of a given study. So, it accounts for the reality that some subjects may drop out of the study before the event of interest happens, or that the study may end before all of the subjects experience the event (time-to-event analysis).
Hazard ratios are calculated via the survival analysis statistical method. In this method, the hazard is the slope of the survival curve, which is a measure of how rapidly subjects are dying. In the general case, this method is used to study the rate of any type of event either negative or positive (not only death). The Hazard Ratio compares two populations, the one under study (e.g. with disease or after intervention) and one control group. Survival curves for both populations are plotted and hazard is calculated. The ratio of the two hazard values is then the Hazard Ratio. There are several statistical methods for sampling the two populations, plotting the survival curves and estimating hazards.
Risk factors are derived from clinical studies. In the past, various evidence ranking schemes have been used, to appraise quality of evidence, based on study design and methodology utilised. One such grading system is the Oxford Centre for Evidence-Based Medicine (OCEBM), which identifies 5 levels of evidence. From the strongest (Level 1) to the weakest, they include:
- Systematic Review (SR) of randomized trials or nested case-control studies, n-of-1 trial, or observational study with dramatic effect
- Individual randomized trial or (exceptionally) observational study with dramatic effect
- Non-randomized controlled cohort/follow-up study
- Case-series, case-control studies, or historically controlled studies
- Mechanism-based reasoning
OCEBM defines these study types are as follows
A systematic review (SR) is a review with a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research. It may also utilize meta-analytical methods to collate and analyze data from the studies that are included in the systematic review (meta-analysis).
Randomized controlled trial (RCT): An epidemiological experiment where eligible people are randomly allocated to two or more groups. One group receives the intervention (i.e. a new drug) while the control group(s) receive(s) inactive placebo (placebo-controlled trial) or an active comparator (comparative effectiveness trial). The researchers assess what happens to people in each group. Any difference in any of the outcomes can be attributed to the intervention.
Cohort Study A longitudinal study that follows a group of people (cohort) who are, have been, or in the future may be exposed or not exposed, or exposed in different degrees, to a factor or factors hypothesized to influence the incidence of a given disease or other outcome.
Case control study: An observational study of persons with the disease of interest (or any outcome variable) and a suitable control (comparison, reference) group of persons without the disease. The relationship of an attribute to the disease is examined by comparing the diseased and non-diseased with regard to how frequently the attribute is present or, if quantitative, the levels of the attribute, in each of the groups.
Case-series: A group or series of case reports involving patients who were given similar treatment. Reports of case series usually contain detailed information about the individual patients. This includes demographic information (for example, age, gender, ethnic origin) and information on diagnosis, treatment, response to treatment, and follow-up after treatment.
A more thorough treatment of the concept of risk factor as perceived in CARRE can be found in Deliverables D.2.1 and D.2.2, available from the project site (www.carre-project.eu).
Author: Kalliopi Pafili, Eleni Kaldoudi, DUTH
Date: 18 March 2014